Worldwide shipping
Shipping & Returns
Quality assured
Find retail locations
Expert support
For All Your Questions
Fast and secure
For All Your Questions
In simple words:
Leak rate = pressure loss over time × box volume.
Unit: mbar·L/s
Example:
Leak rate = (1 mbar × 1000 L) / 1000 s = 1 mbar·L/s → too high.
You want numbers much smaller than that.
Typical practical targets:
| Use case | Leak rate (mbar·L/s) | Meaning |
|---|---|---|
| General R&D / teaching | ≤ 1×10⁻² | Fine with a healthy purifier |
| Battery / advanced materials | ≤ 5×10⁻³ | Faster recovery after transfers |
| Very air-sensitive work | ≤ 1×10⁻³ | Plus strict transfer discipline |
If you are better than 1×10⁻² mbar·L/s and still suffering, look at transfers / solvents / purifier first.
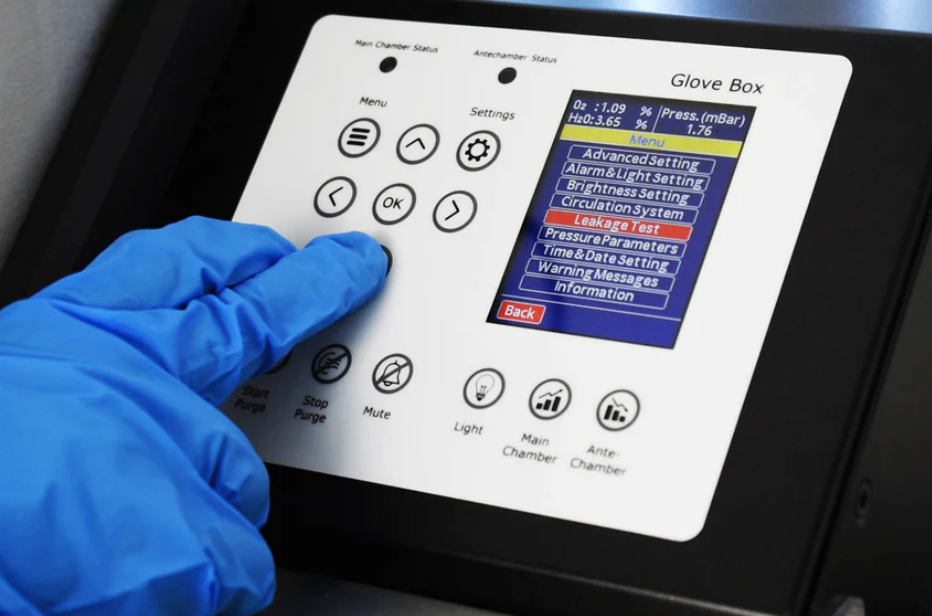
You need:
Steps:
Very common情况:
常见原因:
No account yet?
Create an Account